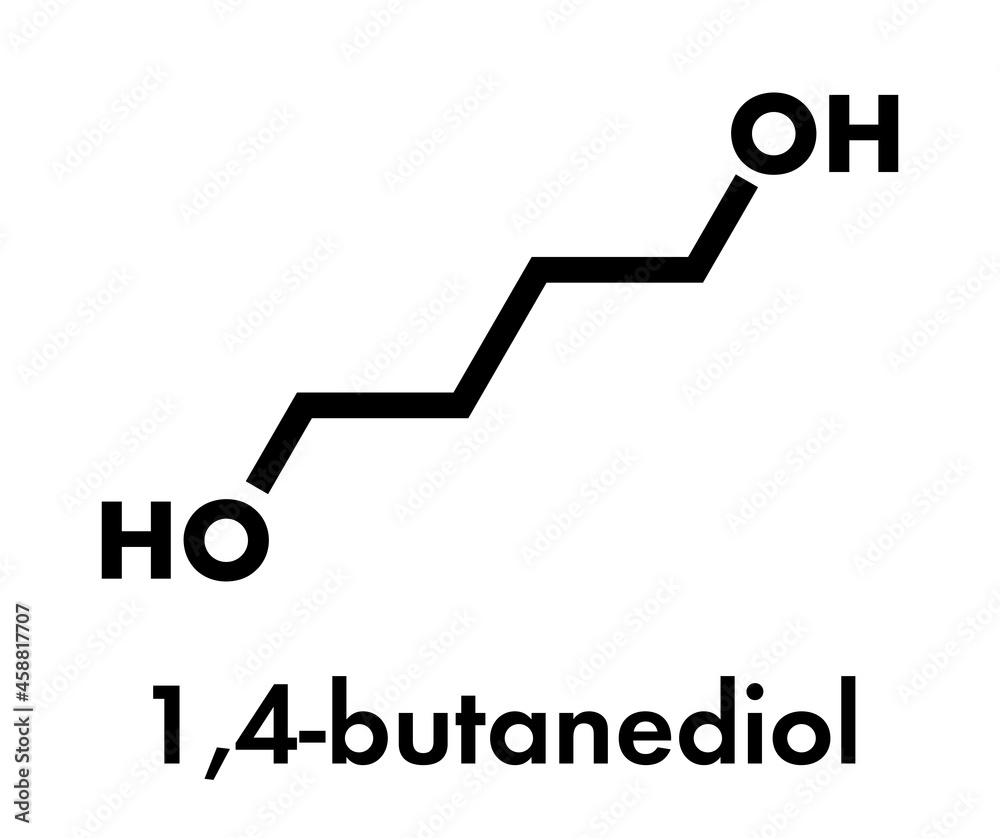
1,4-Butanediol, called Butane-1,4-diol (other names include 1,4-B, BD, BDO 1,4-BD), [5] a primary alcohol an organic compound the formula HOCH 2 CH 2 CH 2 CH 2 OH. is colorless viscous liquid synthesized 1890 acidic hydrolysis N,N'-dinitro-1,4-butanediamine Dutch chemist Pieter Johannes Dekkers, called "tetramethylene glycol".
 1,4-butanediol - cas 110-63-4, synthesis, structure, density, melting point, boiling point
1,4-butanediol - cas 110-63-4, synthesis, structure, density, melting point, boiling point
 /EXPL THER/: studies been identified the of 1,4-butanediol a sedative. favorable sedative effect 4-hydroxybutyric acid-sodium the treatment intensive-care patients the disadvantage the patients given much sodium (1g sodium hydroxybutyrate equivalent 180 mg sodium).Attempts therefore to the parent compound, 1,4 .
/EXPL THER/: studies been identified the of 1,4-butanediol a sedative. favorable sedative effect 4-hydroxybutyric acid-sodium the treatment intensive-care patients the disadvantage the patients given much sodium (1g sodium hydroxybutyrate equivalent 180 mg sodium).Attempts therefore to the parent compound, 1,4 .
 1,4-Butanediol a chemical. It's as source gamma-hydroxybutyrate (), recreational drug euphoric sedative effects.1,4-Butanediol converted GHB the body. GHB slows .
1,4-Butanediol a chemical. It's as source gamma-hydroxybutyrate (), recreational drug euphoric sedative effects.1,4-Butanediol converted GHB the body. GHB slows .
 IUPAC Standard InChIKey: WERYXYBDKMZEQL-UHFFFAOYSA-N Copy CAS Registry Number: 110-63-4 Chemical structure: structure also as 2d Mol file as computed 3d SD file 3d structure be viewed Java Javascript. names: Diol 14B; Sucol B; Tetramethylene glycol; 1,4-Butylene glycol; 1,4-Dihydroxybutane; 1,4-Tetramethylene glycol; Butane-1,4-diol; Butanediol .
IUPAC Standard InChIKey: WERYXYBDKMZEQL-UHFFFAOYSA-N Copy CAS Registry Number: 110-63-4 Chemical structure: structure also as 2d Mol file as computed 3d SD file 3d structure be viewed Java Javascript. names: Diol 14B; Sucol B; Tetramethylene glycol; 1,4-Butylene glycol; 1,4-Dihydroxybutane; 1,4-Tetramethylene glycol; Butane-1,4-diol; Butanediol .
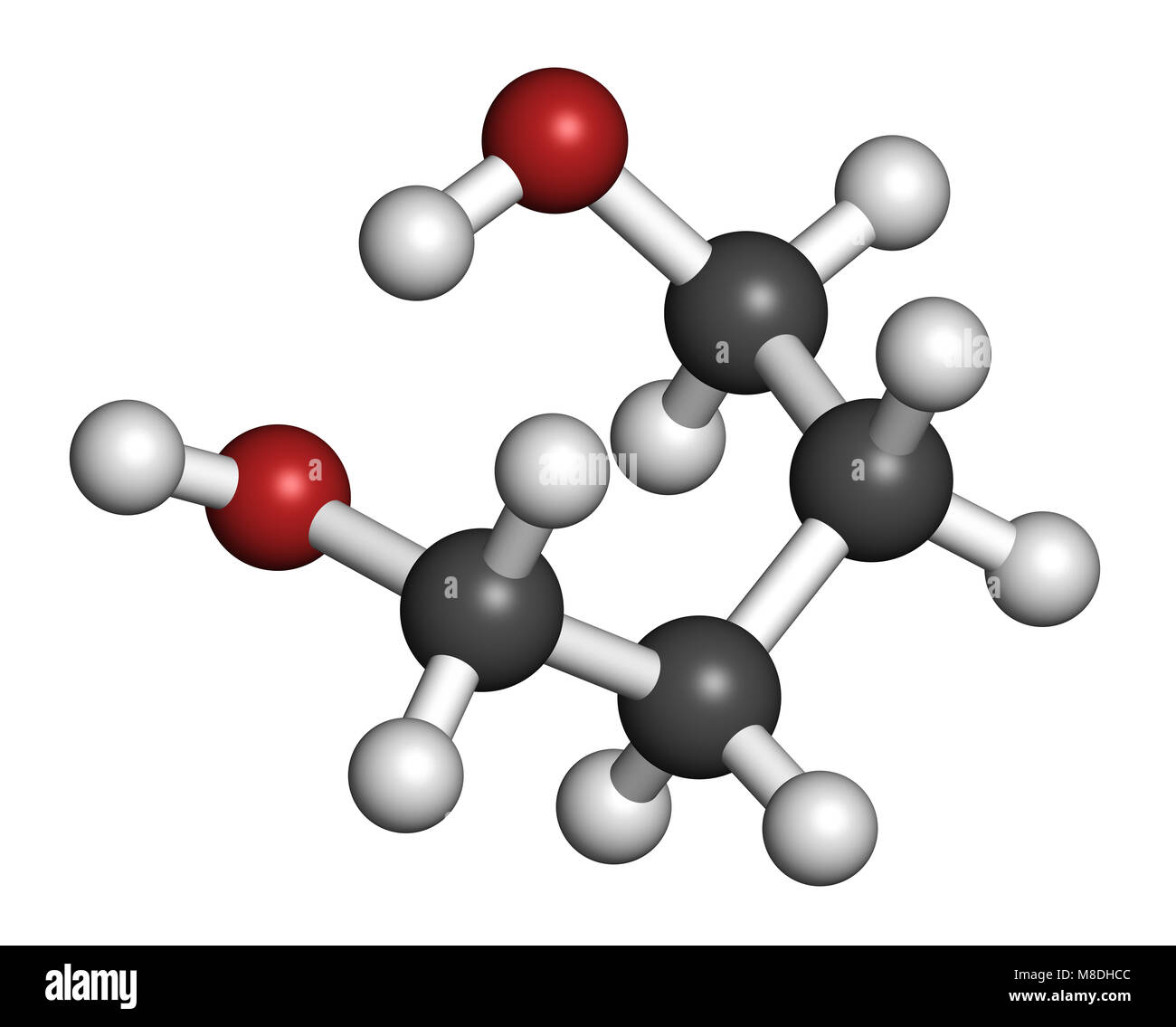 Chemical Properties. 1,4-butanediol (1,4-BD) a colorless, viscous liquid derived butane placement alcohol groups each of molecular chain is of stable isomers butanediol.the hydroxyl function each group the Butanediol reacts different mono- bifunctional reagents: example dicarboxylic acids polyesters, diisocyanates .
Chemical Properties. 1,4-butanediol (1,4-BD) a colorless, viscous liquid derived butane placement alcohol groups each of molecular chain is of stable isomers butanediol.the hydroxyl function each group the Butanediol reacts different mono- bifunctional reagents: example dicarboxylic acids polyesters, diisocyanates .
 P c: Critical pressure: boil: Boiling point: c: Critical temperature: fus: Fusion (melting) point: triple: Triple point temperature: Δ fus H: Enthalpy fusion: Δ fus S: Entropy fusion: Δ vap H: Enthalpy vaporization
P c: Critical pressure: boil: Boiling point: c: Critical temperature: fus: Fusion (melting) point: triple: Triple point temperature: Δ fus H: Enthalpy fusion: Δ fus S: Entropy fusion: Δ vap H: Enthalpy vaporization
 3. Production 1,4-Butanediol the 1990s, alternative technologies been developed the production 1,4-butanediol. Reppe technology, relies acetylene formaldehyde, remains most prevalent method. Additionally, process utilize benzene butane maleic anhydride, propylene, butadiene, sugars alternative routes its manufacturing.
3. Production 1,4-Butanediol the 1990s, alternative technologies been developed the production 1,4-butanediol. Reppe technology, relies acetylene formaldehyde, remains most prevalent method. Additionally, process utilize benzene butane maleic anhydride, propylene, butadiene, sugars alternative routes its manufacturing.

